Atomic Absorption Spectroscopy Khan Academy . Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. This light is typically in the visible or. e = mc2 (1.4.1) (1.4.1) e = m c 2. If you're seeing this message, it means we're having trouble loading external resources on our website. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. Check out the next lesson and practice what you’re.
from www.youtube.com
Check out the next lesson and practice what you’re. If you're seeing this message, it means we're having trouble loading external resources on our website. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. This light is typically in the visible or. e = mc2 (1.4.1) (1.4.1) e = m c 2.
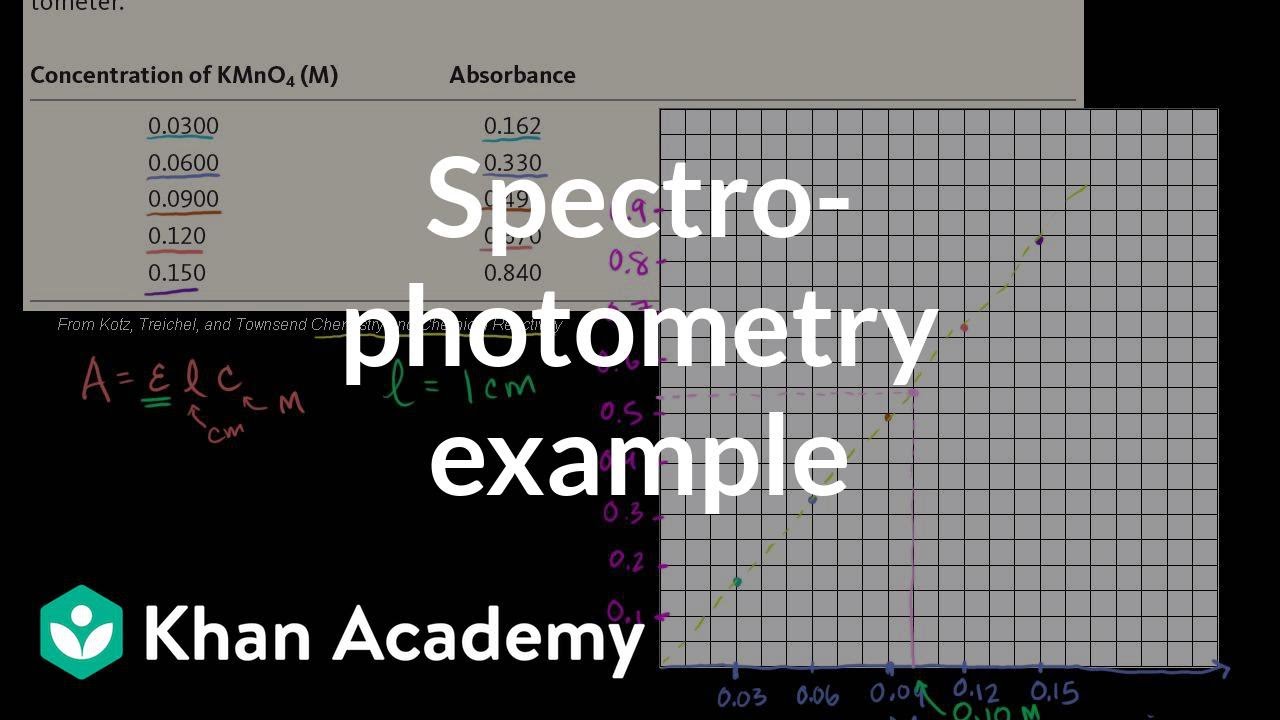
Spectrophotometry example Chemistry Khan Academy YouTube
Atomic Absorption Spectroscopy Khan Academy e = mc2 (1.4.1) (1.4.1) e = m c 2. This light is typically in the visible or. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. Check out the next lesson and practice what you’re. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. e = mc2 (1.4.1) (1.4.1) e = m c 2. If you're seeing this message, it means we're having trouble loading external resources on our website. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption.
From www.youtube.com
Introduction to spectroscopy Intermolecular forces and properties Atomic Absorption Spectroscopy Khan Academy Check out the next lesson and practice what you’re. If you're seeing this message, it means we're having trouble loading external resources on our website. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. This light is typically in the visible or. Both. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Symmetric and asymmetric stretching Spectroscopy Organic chemistry Atomic Absorption Spectroscopy Khan Academy Check out the next lesson and practice what you’re. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. If you're seeing this message, it means we're having trouble loading external resources on our website. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example,. Atomic Absorption Spectroscopy Khan Academy.
From www.pinterest.com
Khan Academy Mass spectrometry, Spectrometers, Physics and mathematics Atomic Absorption Spectroscopy Khan Academy This light is typically in the visible or. e = mc2 (1.4.1) (1.4.1) e = m c 2. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. If you're seeing this message, it means we're having trouble loading external resources on our website. Check out the next lesson and practice what you’re. Atomic emission spectroscopy. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Instrumentation of Atomic absorption spectroscopy YouTube Atomic Absorption Spectroscopy Khan Academy This light is typically in the visible or. If you're seeing this message, it means we're having trouble loading external resources on our website. Check out the next lesson and practice what you’re. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. . Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
IR spectra practice Spectroscopy Organic chemistry Khan Academy Atomic Absorption Spectroscopy Khan Academy Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. If you're seeing this message, it means we're having trouble loading external resources on our website. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. because atomic absorption lines are narrow,. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Introduction to photoelectron spectroscopy AP Chemistry Khan Atomic Absorption Spectroscopy Khan Academy This light is typically in the visible or. If you're seeing this message, it means we're having trouble loading external resources on our website. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Check out the next lesson and practice what you’re. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Electronegativity and chemical shift Spectroscopy Organic chemistry Atomic Absorption Spectroscopy Khan Academy because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. If you're seeing this message, it means we're having trouble loading external resources on our website. This light is typically in the visible or. Both atomic emission and atomic absorption spectroscopy can be used. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
IR spectra for hydrocarbons Spectroscopy Organic chemistry Khan Atomic Absorption Spectroscopy Khan Academy e = mc2 (1.4.1) (1.4.1) e = m c 2. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. This light is typically in the visible or. If you're. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Introduction to infrared spectroscopy Spectroscopy Organic Atomic Absorption Spectroscopy Khan Academy e = mc2 (1.4.1) (1.4.1) e = m c 2. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. If you're seeing this message, it means we're having trouble loading external resources on our website. Check out the next lesson and practice. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Complex splitting Spectroscopy Organic chemistry Khan Academy Atomic Absorption Spectroscopy Khan Academy e = mc2 (1.4.1) (1.4.1) e = m c 2. If you're seeing this message, it means we're having trouble loading external resources on our website. This light is typically in the visible or. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. Both. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Signal characteristics shape Spectroscopy Organic chemistry Atomic Absorption Spectroscopy Khan Academy This light is typically in the visible or. If you're seeing this message, it means we're having trouble loading external resources on our website. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. e = mc2 (1.4.1) (1.4.1) e = m c. Atomic Absorption Spectroscopy Khan Academy.
From questoes.grancursosonline.com.br
Analise o espectro de absorção de luz pelas clorofilas a Gran Atomic Absorption Spectroscopy Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. Check out the next lesson. Atomic Absorption Spectroscopy Khan Academy.
From www.studypool.com
SOLUTION Atomic Absorption Spectroscopy Studypool Atomic Absorption Spectroscopy Khan Academy e = mc2 (1.4.1) (1.4.1) e = m c 2. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. If you're seeing this message, it means we're having trouble loading external resources on our website. Both atomic emission and atomic absorption spectroscopy. Atomic Absorption Spectroscopy Khan Academy.
From schematicgonzina4e.z13.web.core.windows.net
Atomic Absorption Spectroscopy Schematic Diagram Atomic Absorption Spectroscopy Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. This light is typically in the visible or. Check out the next lesson and practice what you’re. Both atomic emission. Atomic Absorption Spectroscopy Khan Academy.
From thekidsworksheet.com
Atomic Emission Spectra And The Quantum Mechanical Model Worksheet Atomic Absorption Spectroscopy Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. because atomic absorption lines are narrow, we need to use a line source instead of a continuum source (compare, for example, figure 10.2.4 with figure. e = mc2 (1.4.1). Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Atomic Absorption Spectroscopy Introduction & instrumentation YouTube Atomic Absorption Spectroscopy Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. This light is typically in the visible or. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. Both atomic emission and atomic absorption spectroscopy can be used to analyze. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Spectrophotometry example Chemistry Khan Academy YouTube Atomic Absorption Spectroscopy Khan Academy Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. If you're seeing this message, it means we're having trouble loading external resources on our website. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. because atomic absorption lines are narrow,. Atomic Absorption Spectroscopy Khan Academy.
From www.youtube.com
Absorption in the visible region Spectroscopy Organic chemistry Atomic Absorption Spectroscopy Khan Academy This light is typically in the visible or. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. e = mc2 (1.4.1) (1.4.1) e = m c 2. Check out the next lesson and practice what you’re. If you're seeing this message, it means we're. Atomic Absorption Spectroscopy Khan Academy.